- First-in-class drug product targeting SGLT-2 in the posterior eye
- Confirmation of drug delivery and efficacy in diabetic primate and rodent models during preclinical phase
SEOUL, South Korea, Sept. 25, 2023 /PRNewswire/ -- Daewoong Therapeutics, a biopharmaceutical company based in South Korea, is pioneering the development of the world's first-in-class eyedrop formulation for the treatment of diabetic eye diseases.
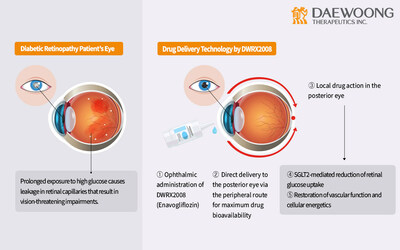
On September 14th, Daewoong confirmed the approval from the Ministry of Food and Drug Safety (MFDS) for a phase 1 clinical trial plan for its investigational product DWRX2008. The inaugural study, set for Q4 of 2023, will assess the safety and pharmacokinetics of DWRX2008 across diverse dosages in both healthy Korean and Caucasian subjects.
DWRX2008 is an innovative nanoparticle eyedrop adaptation of Envlo®(enavogliflozin), which tackles the challenge of poor retinal drug absorption from oral use. It is thought to maintain the dosage efficiency of enavogliflozin, delivering therapeutic effects at just 1/30th the dose of leading SGLT2 inhibitors. Last April, DWRX2008 was granted Korea Drug Development Fund(KDDF) designation for advanced preclinical development. Notably, recent findings indicate its effectiveness in reducing central macular thickness and vascular leakage exclusively in the eyes of diabetic primates that received the treatment, with no such effects observed in untreated eyes.
Diabetic retinopathy, a microvascular complication arising from hyperglycemia and oxidative stress, remains a leading cause of global blindness. While anti-VEGF eye injections serve as the primary treatment and command a $9 billion market, their costs and invasive nature highlight the need for a more affordable and patient-friendly alternative to complement existing therapies.
Bokki Kang, CEO of Daewoong Therapeutics, stated, "DWRX2008 offers a hopeful solution for persistent posterior eye diseases, particularly for those hesitant or unresponsive to eye injections. We're also exploring combination treatment options." He added, "Our dedication to advancing drug delivery technology remains strong as we aim to meet diverse medical needs and uplift patient care worldwide."
Forward-Looking Statements
This press release contains forward-looking statements based on Daewoong Therapeutics' current beliefs and expectations. Factors such as regulatory approvals and clinical trial outcomes may impact the company's business and results, with potential delays and uncertainties in the approval process. The success of their products relies on clinical trial results, and early trials may not predict later-stage outcomes accurately.
Photo - https://mma.prnewswire.com/media/2219948/Photo_Release__DWRX2008_final.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/daewoong-therapeutics-gets-mfds-nod-for-phase-1-ind-for-the-worlds-first-eyedrop-treatment-for-diabetic-retinopathy-and-macular-edema-301937366.html
View original content:https://www.prnewswire.co.uk/news-releases/daewoong-therapeutics-gets-mfds-nod-for-phase-1-ind-for-the-worlds-first-eyedrop-treatment-for-diabetic-retinopathy-and-macular-edema-301937366.html
 Voltar noticias em Inglês
Voltar noticias em Inglês